FieldStrength MRI magazine
Research - December 2017
Confidence in differentiating low and high-grade brain tumors
Physicians at Phoenix Children’s Hospital (PCH) have been studying the value of APT in clinical practice to investigate to what extent APT weighted imaging could be used in the diagnostic and post-therapy imaging of children with brain tumors. Their results suggest APT weighted imaging has the potential to provide higher confidence in determining both the grade of tumor and the extent of residual tumor post-surgery. Many treatment pathways rely on accurate determination of the aggressiveness or “grade” of tumors for the optimal selection amongst treatment options to offer the best possible care choice for patients.
Amide Proton Transfer (APT) weighted imaging is an emerging MRI method that generates image contrast different from conventional MRI. APT weighted imaging is a chemical exchange saturation transfer (CEST) MRI method and its signal is based on the concentration of endogenous proteins and peptides typically present in high-grade brain tumor tissue. Therefore, APT weighted imaging does not require any contrast agent administration.
“Some high-grade tumors demonstrate no gadolinium enhancement and certain low-grade tumors occasionally enhance”

John Curran, MD
Pediatric neuroradiologist and Director of Pediatric Neuroradiology, Phoenix Children's Hospital. His interests include imaging of pediatric brain tumors and imaging of epilepsy.

Jeffrey H. Miller, MD
Pediatric neuroradiologist and Vice Chair of Radiology for Research and Academic Affairs at Phoenix Children’s Hospital. His academic affiliations include Clinical Assistant Professor of Radiology, University of Arizona College of Medicine, the Mayo Clinic-Scottsdale and the Barrow Neurological Institute at Phoenix Children’s Hospital. His current research interests are functional MRI, MR connectomics, and brain PET-CT. He has lectured extensively on advanced pediatric MR imaging.
Looking for improved confidence in brain tumor diagnoses
MRI is widely used for visualizing primary brain tumors and secondary lesions in oncology patients. Its excellent soft tissue contrast and functional imaging provide radiologists information on the location, size, morphology, composition and physiology of lesions to help them in diagnosing and staging. Still, there are cases where radiologists would like to have additional capabilities for their diagnosis, for instance in distinguishing high-grade and low-grade tumors with more confidence and ultimately for performing the numerous follow-up MRI exams without contrast administration in children after brain tumor resection. In the United States alone, nearly 80,000 new cases of primary brain tumor are expected to be diagnosed in 2017, including more than 26,000 primary malignant brain tumors.[1] Gliomas represent 75% of all malignant tumors, and 55% of these are glioblastoma with 12,930 cases predicted for 2017.[1,2] Given that incidence, and the impact of the correct diagnosis and appropriate treatment paths, oncologists and radiologists weclome
innovative tools to support their current means and strategies. One of these may be adding APT weighted imaging to the MRI exam. APT contrast correlates with the presence of proteins and peptides that may be related to cell proliferation. Since cell proliferation is a feature of tumors, APT color maps can be useful in identifying and quantifying tumor tissue.[3,4]

Phoenix Children’s Hospital
APT reflects concentration of endogenous proteins in brain tumor
In APT weighted imaging and other CEST methods, the MRI signal is generated by a mechanism different from that of basic MRI. These CEST techniques are based on the chemical exchange of hydrogen atoms. The signal of amide protons of peptide bonds in proteins is too low to be measured in normal MRI. The hydrogen (proton) exchange between protein amide groups and surrounding water allows a different way to measure these amide protons. In APT a narrow RF prepulse (saturation pulse) at the amide hydrogen’s frequency is given to attenuate its MR signal. Because the amide group and water continually exchange hydrogen atoms, the number of saturated protons will build up in water, so that the measured water signal will become lower. The change of the MRI signal of water provides an indirect way to measure the presence of amide. APT images are usually presented as color maps, created by using an asymmetry calculation so that presence of APT is shown as a positive colored signal.
Proteins with amide protons surrounded by water molecules that are moving around.
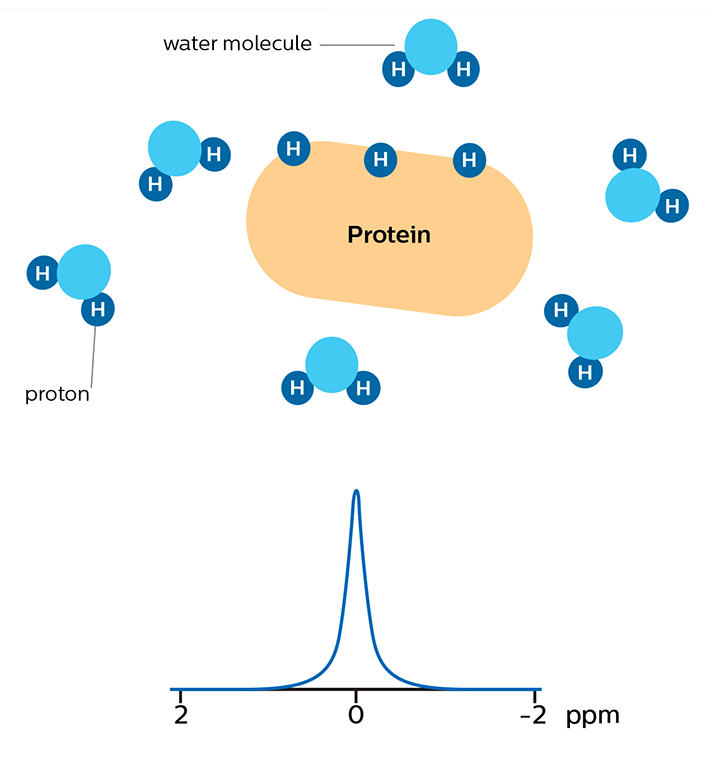
MR signal of water is high
Saturation prepulse on protein's amide proton frequency nulls MR signal of these protons
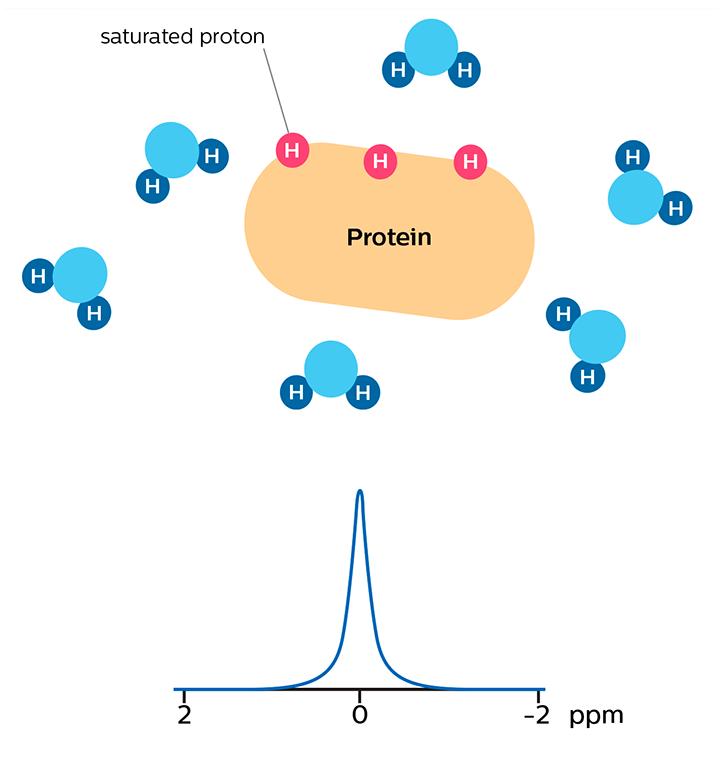
MR signal of water is high
As a result of chemical exchange the nulled protons move from the protein to water molecules.
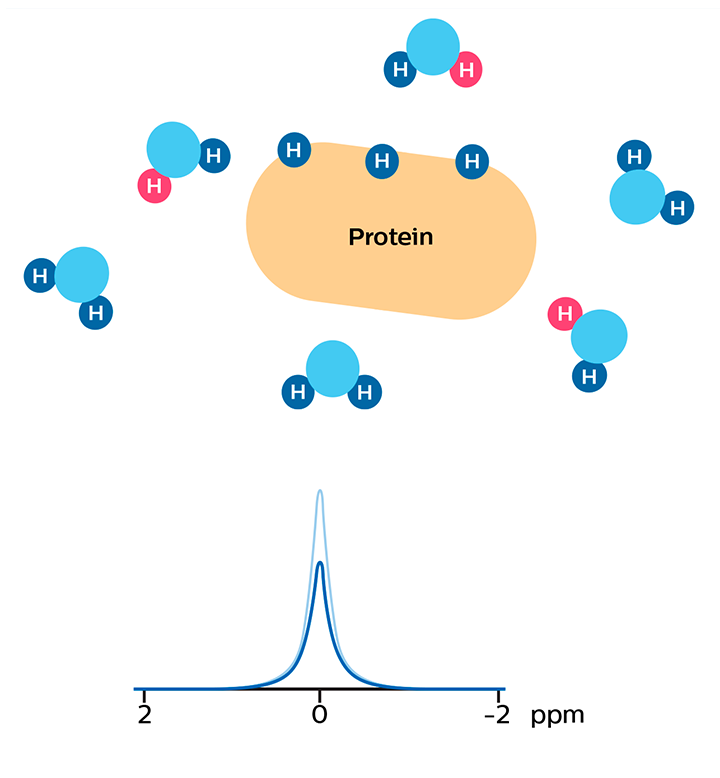
MR signal of water is reduced due to the proton exchange. In APT, this signal change is used to calculate an APT map that is sensitive to the concentration of the protein.
Studies have shown that the APT signal correlates with the concentration of a protein that is related to cell proliferation. The concentration of this protein, and thus the APT signal strength responds to the grade of malignant tumors.[5-7] APT contrast can potentially highlight tumors that wouldn’t be seen otherwise.
"Some high-grade tumors demonstrate no gadolinium enhancement and certain low-grade tumors occasionally enhance"
Tumor grading can affect critical decision making The choice of treatment paths often strongly depends on the tumor grade. Common treatment options for high-grade tumors include surgical tumor resection followed by additional therapy such as radiation and or chemotherapy. Quick and decisive action is desirable in these cases as median survival for glioblastoma, for instance, is between 12.6 and 14.6 months, although longer rates have been reported.[8,9] Given the lower tumor growth rate of low-grade tumors, a range of potential treatment options exist for these cases. The selection of the most appropriate treatment is based on the balance of therapeutic benefits and side effects. At times, surveillance imaging may play a role while the choices of definitive therapy are being considered.[10] MR imaging is often used by radiologists and physicians in estimating the grade of brain tumors, but there is sometimes still uncertainty.[9,11] Differentiating between low-grade and high-grade tumors is not straightforward, even for the highly experienced radiologist. Gadolinium enhancement is not always specific for tumor grade, as some high-grade tumors demonstrate no gadolinium enhancement and certain low-grade tumors occasionally enhance (e.g. DNET). Gadolinium enhancement also occurs in any area of a blood-brain barrier disruption, such as treatment-related injury.[12] The power of APT for grading brain tumors with MRI While the gold standard for grading of gliomas is histopathology after biopsy, MRI is often used in monitoring glioma patients, and APT can be a valuable addition to the MRI exam in these patients. Tumor grade and APT signal have been observed to be commonly positively correlated: high-grade tumors tend to exhibit a high APT contrast.[12-15] APT images can be seen to visualize tumor with more emphasis than post-contrast images, resulting in a scan that may be easier to interpret. Scientific studies comparing tumor grades with APT signal in adult glioma suggest that APT can support tumor grading, separating high-grade from low-grade, even when traditional MRI is inconclusive.[5,13,14]
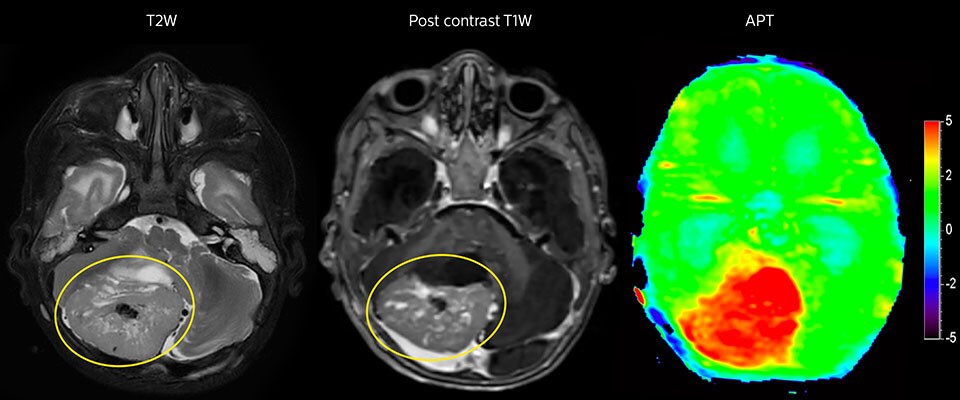
APT imaging of high-grade tumor
Tumor evaluation in a 1-year-old child with medulloblastoma. This aggressive tumor type is very solid and homogeneous. The high APT signal corresponds with the post-contrast image of this high-grade tumor.
APT may be a powerful addition to grade tumors with MRI
Dr. Jeffrey Miller, pediatric radiologist at PCH also noticed the relation between APT contrast and tumor grades in the studies done at his hospital. “In several cases we have seen a high APT signal in high-grade tumors and moderately increased APT signal in cases with intermediate and low-grade tumors that have the characteristic of high signal change on T2 and FLAIR, and no contrast enhancement.” He points out the potential clinical implications of this observation. “When we’re faced with patients where the diagnosis is a little bit ambiguous, we often have to make choices and value judgements, which could mean either just following up the tumor or lesion, with the risk that it could change when we were wrong and there could be time lost. Or we have to go into invasive situations where we have to biopsy.” “It would be very impactful and valuable to have a sequence like APT weighted imaging, which could assist us in making those decisions with more confidence. That would be meaningful for the individual patients and take out some ambiguity in what we are doing.” “However, in order to reach that lofty goal, we will need more investigation, use the sequence in a larger population, and gain more understanding of situations and conditions where APT has its maximal value.”
"It would be impactful to have a sequence like APT, which could assist us in making those decisions with more confidence”
APT may illuminate post-resection images with crucial information
MRI may be performed after tumor resection, to look for residual tumor or tumor regrowth. Also here, the different contrast mechanism of APT may help in diagnosis. Dr. Miller remembers a particular case. “After a very good resection, we saw small changes on the postcontrast T1-weighted and the T2-weighted images that looked like a post-surgical little bit of fluid. Interestingly, however, we saw a focal area of APT signal, right in the center of that abnormality. As we usually do when a bit unsure, we followed it up and, unfortunately, found tumor regrowth in that region,” Dr. Miller says. “Cases like this motivate me, and others who care about this population, to investigate how this APT method could be used on large scale in this population and help us in providing high value diagnostic information.” The hospital’s physicians also saw a case where APT had a negative predictive value. Following the resection of a highgrade tumor, they saw a similar small change in the images of this patient. However in this case, the APT signal was rather low. In a recent rescanning of this patient, no recurrence was seen.
"Such cases motivate me to investigate how APT could be used on large scale''
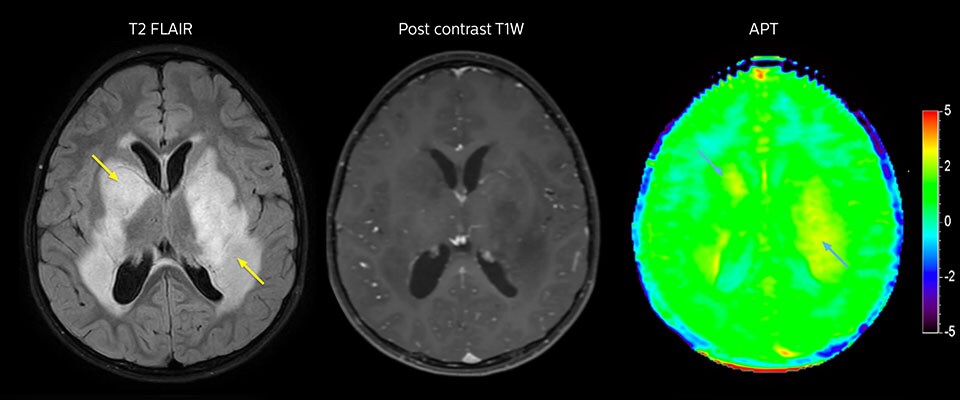
APT imaging of low-grade tumor
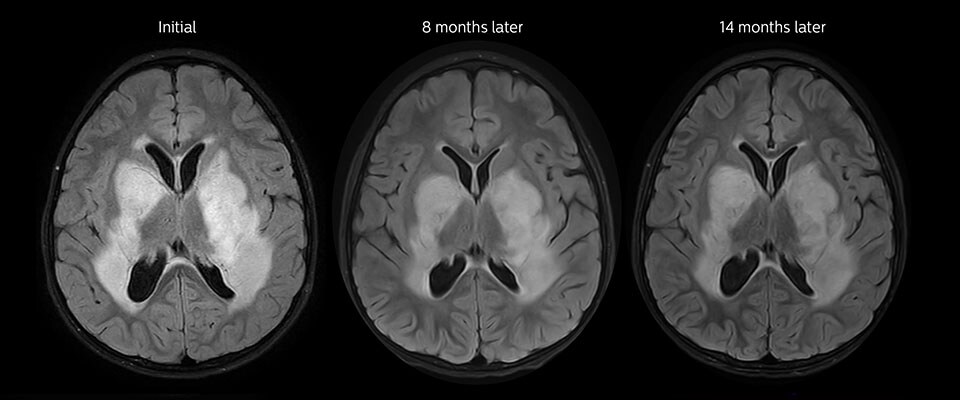
Low-grade glioma in a 5-year-old patient with neurofibromatosis 1. This low-grade lesion does not enhance on the post-contrast images, but does show an intermediate APT signal. The lesion stability over time confirms that it is a low-grade pathology.
Follow-up over time
"In many cases, we have seen that APT is positive when postcontrast T1-weighted imaging is positive"
APT MRI study encouraging for the youngest tumor patients
Radiologist John Curran, MD, has been the main researcher in studying APT weighted imaging at Phoenix Children’s Hospital. “At this time, APT has been added to approximately 70 MRI studies of children with brain tumor and we have seen some encouraging early results,” says John Curran, MD, radiologist at Phoenix Children’s Hospital (PCH). “We will need larger studies with more patients to accurately assert the correlation. However, it doesn’t need to be a 100% correlation to be useful in brain tumor follow-up scans, because we also look at the FLAIR and at other images. The goal is to catch something before it gets too big, if it needs new surgery or new therapy and if we see something suspicious – as opposed to definite recurrence – it is often not a matter for immediate action, but for follow up.”
The PCH physicians involved in the trial overall express cautious optimism that APT weighted imaging may someday greatly reduce the need for contrast injection in pediatric patients. “If we can bring APT forward as a reasonable substitute, particularly in our follow-up brain tumor cases that would be of great benefit,” says Dr. Curran. “Use of contrast agent is tightly controlled in our general neuroradiology imaging, and contrast agent is only administered when it is really needed. So, our study focuses on determining if we in the future could use APT to diminish the use of contrast agents.”
"APT was added to about 70 MRI exams of children with brain tumor and we saw some encouraging early results”
Promising results with APT inspire further plans
Dr. Curran has been comparing APT weighted imaging to postcontrast MRI in children with brain tumor history. “In many cases, we have seen that APT is positive when post-contrast T1-weighted imaging is positive. So, we’ve been trying to assess if that relationship holds up well enough to possibly use APT instead of giving a child contrast agent in certain circumstances.” The study uses APT research software that was developed by Philips in a research collaboration.
“Brain tumor MRI usually includes post-contrast imaging. So, in our young patient population, our concerns relate to the need to administer gadolinium-based contrast agent in follow-up scans in children after brain tumor resection. A study published by my colleague Dr. Miller showed that if a tumor is resected in a young child, by the time that child is at young adult age, an appreciable amount of gadolinium has been deposited in the brain.[16] APT does not require any contrast agent. So, if we can bring APT forward as a reasonable substitute, particularly in our follow-up brain tumor cases, that would be of great benefit.”
APT in post-surgery evaluation
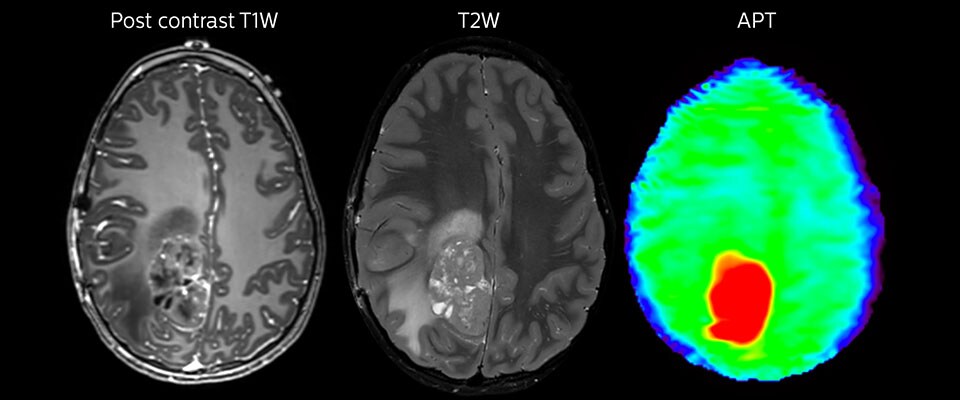
Large metastatic brain lesion
This 10-year-old patient underwent Ewing’s sarcoma tumor resection 7 years ago, but was found to now have a large metastatic lesion in the brain. This lesion shows clearly increased APT signal.
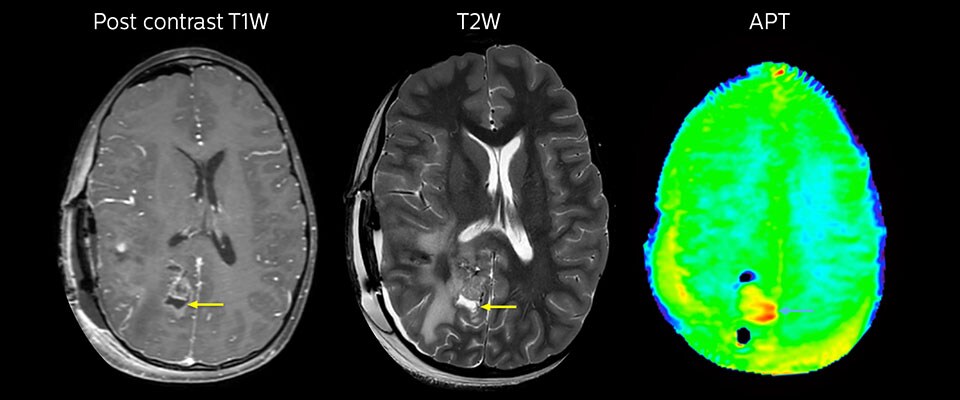
MRI with APT post resection
Immediately post resection MRI was again performed. T2-weighted and postcontrast T1-weighted images are quite inconclusive for distinguishing residual tumor tissue from postoperative tissue changes. On the APT image some high signal is still seen, which would suggest residual tumor tissue.
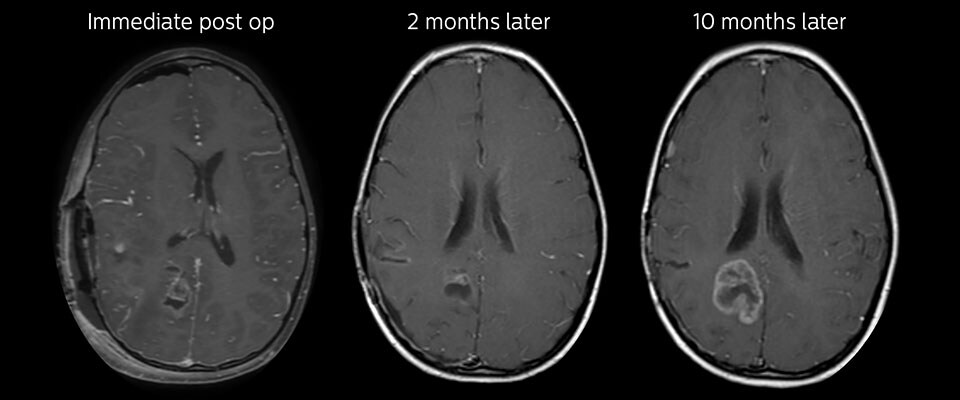
Follow-up over time
In later follow-up scans the post-contrast T1-weighted images suggest recurrent tumor growth. So, it would be interesting to study the predictive value of APT in a large patient group.
Looking forward to further research on the potential of APT
According to Dr. Curran, the main APT research focus at PCH has so far been on investigating its capabilities in visualizing brain tumors and its potential for reducing the need for contrast. “We hope that APT in future can help us in further characterizing tumors with MRI, but more research will need to be done before we fully know what is possible and effective. By looking at specific aspects of tumors that are APT positive, we hope to correlate APT signal to more precise histology or tumor markers.”
“We look forward to in future have a test to help us with the direction of therapy, such as choosing chemotherapy agents, plus or minus radiation and so forth. Perhaps APT may in future have a potential to help us there in some way,” Dr. Curran says. “The possibilities seem very broad.”
Dr. Miller concludes by summarizing “We’ve had some really good experience in using an APT method in a clinical situation. We have learned a lot in the process and see a lot of potential for it in the future.”
"We’ve had some really good experience in using an APT method in a clinical situation"
References
1. American Brain Tumor Association, Brain Tumor Statistics. 9. Lobera, A, Coobs, B, Naul, LG, Zee, CS. Imaging in Glioblastoma Multiforme. Medscape. 13. Park KJ, Kim HS, Park JE et al. Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur Radiol 2016, 26: 4390; doi: 10.1007/s00330- 016-4261-2.
2. Central Brain Tumor Registry of the United States, 2016 CBTRUS Fact Sheet.
3. Togao O, Hiwatashi A, Keupp J, Yamashita K, Kikuchi K, Yoshiura T, Yoneyama M, Kruiskamp MJ, Sagiyama K, Takahashi M, Honda H. Amide Proton Transfer Imaging of Diffuse Gliomas: Effect of Saturation Pulse Length in Parallel Transmission-Based Technique. PLOS ONE 2016; doi: 10.1371/journal.pone.0155925.
4. Togao O, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, Yoneyama M, Honda H. Amide Proton Transfer Imaging of Brain Tumors Using a Self-Corrected 3D Fast Spin-Echo Dixon Method: Comparison with Separate B0 correction Magn Res Med 2016 early view; doi: 10.1002/mrm.26322.
5. Togao O, Yoshiura T, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, Suzuki, YS, Iwak, T, Hata N, Mizoguchi M, Yoshimoto K, Sagiyama K, Takahashi M, Honda H Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-Oncology 2014; 16(3), 441–448.
6. Jiang S, Eberhart CG, Zhang Y, Heo HY, Wen Z, Blair L, Qin H, Lim M, Quinones- Hinojosa A6, Weingart JD, Barker PB, Pomper MG, Laterra J, van Zijl PCM, Blakeley JO, Zhou J. Amide proton transfer-weighted magnetic resonance image-guided stereotactic biopsy in patients with newly diagnosed gliomas. Eur J Cancer 201783:9-18; doi: 10.1016/j.ejca.2017.06.009.
7. Zhou, J. (2011). Amide Proton Transfer Imaging of the Human Brain. Magnetic Resonance Neuroimaging. Methods in Molecular Biology (Clifton, N.J.), 711, 227–237.
8. American Brain Tumor Association, Brain Tumor Information, Types of Tumors, Glioblastoma (GBM)
10. Paleologos, N. Low Grade Glioma: Update in Treatment and Care. ABTA Patient and Family Conference, 2014.
11. Upadhyay, N, Waldman, A D, Conventional MRI evaluation of gliomas. The British Journal of Radiology 2011; 84: S107–S111.
12. Wen, Z, Hu, S, Huang, F, Wang, X, Guo, L, Quan, X, Wang, S, Zhou, J, MR Imaging of High-Grade Brain Tumors Using Endogenous Protein and Peptide- Based Contrast. NeuroImage 20110; 51(2), 616–622.
14. Park JE, Kim HS, Park KJ, Kim SJ, Kim JH, Smith SA. Pre- and Posttreatment Glioma: Comparison of Amide Proton Transfer Imaging with MR Spectroscopy for Biomarkers of Tumor Proliferation. Radiology 2016, 278 :514; doi: 10.1148/ radiol.2015142979.
15. Wang X, Yu H, Jiang S, Wang Y, Wang Y, Zhang G, Jiang C, Song G, Zhang Y, Heo H-Y, Zhou J, Wen Z. Qualitative and quantitative analysis of amide proton transferweighted MR images at 3 Tesla of adult gliomas. Abstract #1105, ISMRM 2017.
16. Miller JH, Hu HH, Pokorney A, Cornejo P, Towbin R. MRI Brain Signal Intensity Changes of a Child During the Course of 35 Gadolinium Contrast Examinations. Pediatrics 2015;136;e1637.

More from FieldStrength