- IVUS helps disease assessment
-
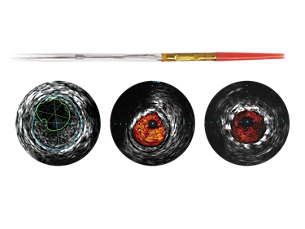
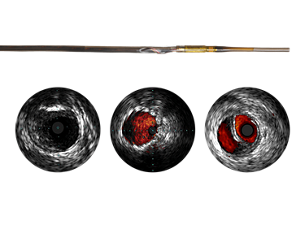
IVUS helps disease assessment
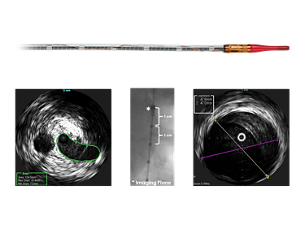
IVUS imaging helps physicians assess disease markers, including plaque burden percentage, lesion location and morphology, calcium volume and the presence of thrombus. It also provides analysis of crucial parameters—like luminal cross-sectional measurements—and aids in disease diagnosis. - Helps intervention decisions
-
Helps intervention decisions
Not only does IVUS provide real-time diagnostic imaging for peripheral artery disease, it may also guide clinicians to the correct angioplasty technique for the patient’s individual needs, assess intervention effectiveness and assist in endovascular device delivery. The imaging modality also helps clinicians decide the size of the device needed for the best outcome. - Confirm treatment results
-
Confirm treatment results
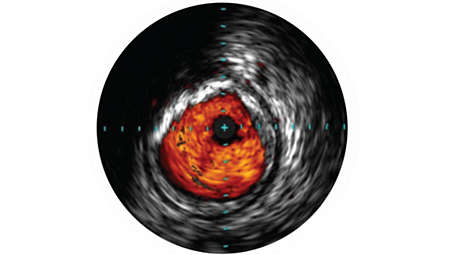
IVUS imaging helps to confirm treatment results, including the completeness of treatment, the apposition and expansion of stent placement, and whether or not the patient requires a thrombolytic drip. ChromaFlo imaging, for example, can be used to show evidence of stent apposition by illustrating the absence of flow. - Stent apposition assessment
-
Stent apposition assessment
ChromaFlo highlights blood flow red for easy assessment of stent apposition, lumen size, and more. Appropriate for peripheral and coronary vessels, including left main, bifurcations, superficial femoral artery, and iliac. It is designed to make lumen size and stent apposition instantly recognizable and helps identify branches, dissections, and plaque in bifurcations.
IVUS helps disease assessment
IVUS helps disease assessment

IVUS helps disease assessment
Helps intervention decisions
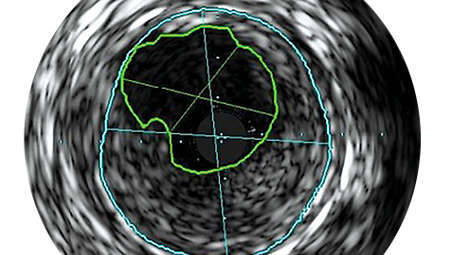
Helps intervention decisions

Helps intervention decisions
Confirm treatment results

Confirm treatment results

Confirm treatment results
Stent apposition assessment
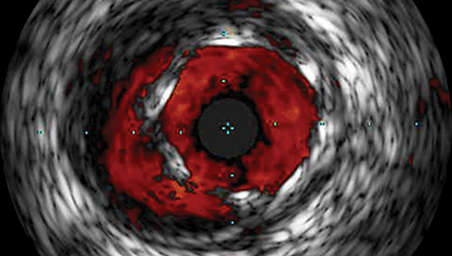
Stent apposition assessment

Stent apposition assessment
- IVUS helps disease assessment
- Helps intervention decisions
- Confirm treatment results
- Stent apposition assessment
- IVUS helps disease assessment
-
IVUS helps disease assessment
IVUS imaging helps physicians assess disease markers, including plaque burden percentage, lesion location and morphology, calcium volume and the presence of thrombus. It also provides analysis of crucial parameters—like luminal cross-sectional measurements—and aids in disease diagnosis. - Helps intervention decisions
-
Helps intervention decisions
Not only does IVUS provide real-time diagnostic imaging for peripheral artery disease, it may also guide clinicians to the correct angioplasty technique for the patient’s individual needs, assess intervention effectiveness and assist in endovascular device delivery. The imaging modality also helps clinicians decide the size of the device needed for the best outcome. - Confirm treatment results
-
Confirm treatment results
IVUS imaging helps to confirm treatment results, including the completeness of treatment, the apposition and expansion of stent placement, and whether or not the patient requires a thrombolytic drip. ChromaFlo imaging, for example, can be used to show evidence of stent apposition by illustrating the absence of flow. - Stent apposition assessment
-
Stent apposition assessment
ChromaFlo highlights blood flow red for easy assessment of stent apposition, lumen size, and more. Appropriate for peripheral and coronary vessels, including left main, bifurcations, superficial femoral artery, and iliac. It is designed to make lumen size and stent apposition instantly recognizable and helps identify branches, dissections, and plaque in bifurcations.
IVUS helps disease assessment

IVUS helps disease assessment

IVUS helps disease assessment
Helps intervention decisions

Helps intervention decisions

Helps intervention decisions
Confirm treatment results

Confirm treatment results

Confirm treatment results
Stent apposition assessment

Stent apposition assessment

Stent apposition assessment
Documentation
-
Brochure (2)
-
Brochure
- Product Brochure (2.4 MB)
- Product Brochure (1.3 MB)
-
Brochure (2)
-
Brochure
- Product Brochure (2.4 MB)
-
Brochure (2)
-
Brochure
- Product Brochure (2.4 MB)
- Product Brochure (1.3 MB)
Specifications
- Reconnaissance PV .018 OTW
-
Reconnaissance PV .018 OTW Minimum guide catheter - 5F
Maximum imaging diameter - 20 mm
Frequency - 0.071 in
Frequency - 20 MHz
Maximum guide wire - 0.018 in
Working length - 150 cm
-
- Reconnaissance PV .018 OTW
-
Reconnaissance PV .018 OTW Minimum guide catheter - 5F
Maximum imaging diameter - 20 mm
-
- Reconnaissance PV .018 OTW
-
Reconnaissance PV .018 OTW Minimum guide catheter - 5F
Maximum imaging diameter - 20 mm
Frequency - 0.071 in
Frequency - 20 MHz
Maximum guide wire - 0.018 in
Working length - 150 cm
-
Related products
Alternative products
-
Visions PV .014P RX
- Digital IVUS catheter evaluates vascular morphology in blood vessel
- Provides 55% increase pushability over PV .014P digital IVUS catheter
- 150 cm working length, 20 mm max imaging diameter for .014” guide wire interventional procedures
- Grayscale IVUS, VH IVUS and ChromaFlo capable*
View product
-
Visions PV .035
- Digital IVUS catheter evaluates vascular morphology in blood vessels
- Provides cross-sectional imaging of these vessels
- 90 cm length and 60 mm max imaging diameter for 0.035” guide wire interventional procedures
- Grayscale IVUS capable
View product
-
Visions PV .018
- Digital IVUS catheter evaluates vascular morphology in blood vessels
- Provides cross-sectional imaging of these vessels
- 135 cm working length and 24 mm max imaging diameter for 0.018” guide wire interventional procedures
- Grayscale IVUS and ChromaFlo capable
View product
-
Visions PV .014P RX
The Visions PV .014P RX digital IVUS catheter has a 55% stiffer shaft than Eagle Eye Platinum and PV .014P digital IVUS catheters to facilitate greater pushability while preserving the equivalent level of trackability.¹ IVUS provides detailed and accurate assessment of lumen size, vessel size, plaque morphology and key anatomical landmarks.
View product
-
Visions PV .035
As an adjunct to conventional angiographic interventions, the Visions PV .035 digital IVUS catheter evaluates vascular morphology in blood vessels and provides cross-sectional imaging of these vessels. With a 90 cm length and 60 mm max imaging diameter for 0.035” guide wire interventional procedures, the device aids in peripheral artery disease diagnosis and venous disease and guides clinicians toward the correct therapy for the patient’s unique needs.
View product
-
Visions PV .018
As an adjunct to conventional angiographic interventions, the Visions PV .018 digital IVUS catheter evaluates vascular morphology in blood vessels and provides cross-sectional imaging of these vessels. With a 135 cm working length and 24 mm max imaging diameter for 0.018” guide wire interventional procedures, the device aids in peripheral artery disease diagnosis and guides clinicians toward the correct therapy for the patient’s unique needs.
View product
- *Safety and effectiveness of VH IVUS for use in the characterization of vascular lesions and tissue types has not been established.
- 1. Nair A, Margolis M, Kuban B, Vince D. Automated Coronary Plaque Characterisation with Intravascular Ultrasound Backscatter: Ex Vivo Validation. EuroIntervention. 2007; 3: 113-120.
- Product availability is subject to country regulatory clearance. Please contact your local sales representative to check the availability in your country.
- Always read the label and follow the directions for use.
- Philips medical devices should only be used by physicians and teams trained in interventional techniques, including training in the use of this device.
- Philips reserves the right to change product specifications without prior notification.
- ©2025 Koniklijke Philips N.V. All rights reserved. Trademarks are the property of Koninklijke Philips N.V. or their respective owners.