Related products
Alternative products
-
Incisive CT
- Elevates your business with Tube for Life guarantee
- Delivers intelligence that adapts to you with OnPlan patient-side gantry controls
- Brings predictability to an unpredictable world with Performance Bridge
View product
-
MR 7700
- XP gradients with high accuracy, power, and endurance
- Facilitate the most demanding research programs
- Supporting confident diagnosis for every patient
View product
-
Ingenia Elition 3.0T X
- A revolutionary breakthrough in diagnostic quality and speed
- Setting new directions for clinical and research 3.0T imaging
- Based on gradient designs with high accuracy, power, and endurance
View product
-
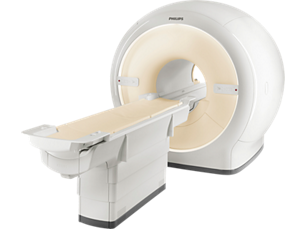
Ingenia 1.5T
- Embrace emerging applications
- Welcome challenging patients
- Premium IQ⁴ with digital clarity and speed
- Personalize your imaging
View product
-
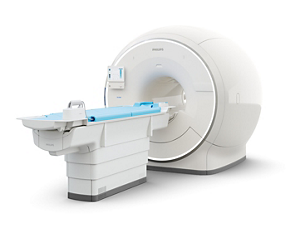
MR 5300
- Quickly, Easily and Confidently.
- Powered by Philips exclusive BlueSeal magnet
View product
-
Incisive CT
Incisive CT helps you meet some of your organization’s most pressing challenges. Philips Incisive CT offers intellect at every step, from acquisition through results, and across all fronts: financial, clinical and operational. Like never before, operator and design efficiencies come together for wise decisions from start to finish with an unprecedented Tube for Life guarantee¹. Now with the CT Smart Workflow, Incisive CT has further differentiated itself. CT Smart Workflow is an entirely new package of AI enabled tools that bring you the industry’s fastest AI reconstruction, automatic patient positioning and so much more to aid successful exams with fast results at low dose. From motion-free cardiac imaging to interventional procedures with confidence, CT Smart Workflow offers you advances that matter in your day-to-day imaging.
View product
-
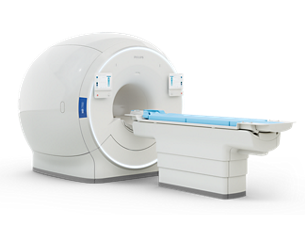
MR 7700
Experience breakthrough innovation in 3.0T imaging with the unique design of the Philips MR 7700 imaging system, enhanced with XP gradients and artificial intelligence (AI). The system is built to address a pressing need to deliver on the clinical expectations of today, and to facilitate the most demanding research programs. The MR 7700 provides high accuracy, power, and endurance to support confident diagnosis for every patient. It is the system of choice for highest quality diffusion imaging and advanced neuroscience. Extend your scanning capabilities with a fully integrated multi-nuclei imaging and spectroscopy solution to explore new clinical pathways without sacrificing clinical imaging workflow or wide-bore patient comfort. What’s more? The MR 7700 promises a great experience for both users and patients through the ease-of-use features of a well-designed clinical 3.0T scanner together with a no compromise workflow. Now scientists and clinicians alike can schedule without conflict.
View product
-
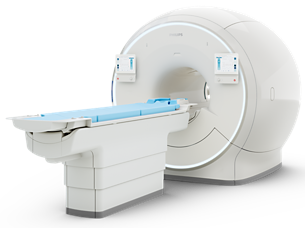
Ingenia Elition 3.0T X
The Philips Ingenia Elition solution offers cutting-edge MR imaging techniques, while setting new standards for clinical research in 3.0T imaging based on gradient- and RF designs. The Ingenia Elition delivers on superb image quality, and performs MRI exams up to 50% faster¹. Fast overall exam-time is achieved by improving patient handling setup time at the bore with the touchless guided patient setup, combined with accelerations in both 2D- and 3D scanning. Furthermore, the Ingenia Elition offers an immersive audio-visual experience to calm patients and guide them through MR exams.
View product
See all related products -
Ingenia 1.5T
Put quality first with Philips Ingenia 1.5T MRI system. Digital clarity and speed¹ help clinicians diagnose with confidence, explore new applications, and work productively. Great patient reviews build your image in the community. All supported by our commitment to helping you grow.
View product
-
MR 5300
This innovative 1.5T MRI system is powered by Philips exclusive BlueSeal magnet for helium-free operations. And it incorporates a wealth of AI²-driven technologies to simplify and automate the most complex clinical and operational tasks. So you can focus on what matters the most: your patients. This breakthrough solution is designed to help boost MR productivity, speed up exams, empower clinicians to make informed clinical decisions, and control the costs of MR imaging.
View product