Precise, personalized care starts here
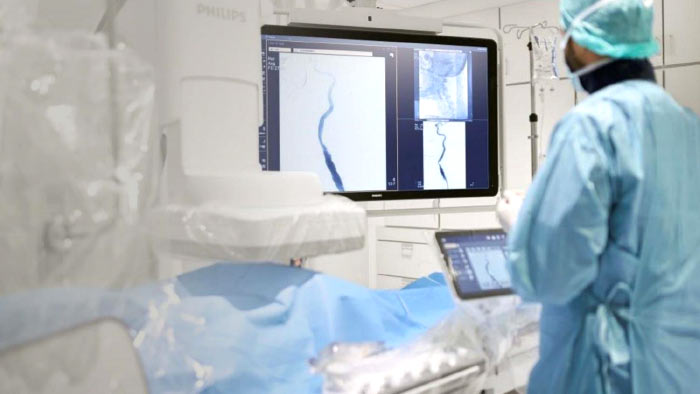
Early and accurate diagnosis leads to clear options for treatment. Unfortunately, standard TRUS prostate biopsies are often imprecise, with the ability to detect only 30-41% of cancers.1 Philips offers a comprehensive prostate cancer diagnosis solution in utilizing MRI/US fusion that is in use at 22 of the 25 of the top cancer hospitals in the US.2 This unified, orchestrated approach to prostate cancer diagnosis and care has significant benefits for clinical teams and for patients.
This more targeted approach can benefit patients by potentially reducing instances of biopsy-related hospitalization, decreasing likelihood of sepsis, and most importantly, avoiding the need for repeat biopsies.3 Whichever method is chosen, ensuring that patients have the highest quality care possible can be complicated given the many specialties involved in the prostate cancer journey, such as urology, radiology, pathology, genomics and oncology. Inefficient and disconnected workflows further complicate the picture.
Did you know?

Troubling inequities
Research suggests that prostate cancer is most prevalent in men of African descent, compared to men of European or Asian descent8
Key capabilities for clarity in prostate cancer diagnosis
-
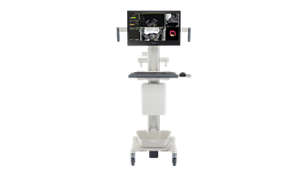
UroNav
UroNav fuses pre-biopsy MR images of the prostate with ultrasound-guided biopsy images in real time, for excellent delineation of the prostate and suspicious lesions, as well as clear visualization of the biopsy needle path. Combining electromagnetic tracking and navigation with an onboard computer and a real-time imaging interface, UroNav brings precision targeting to your clinical practice in one easy-to-use, mobile workstation.
784026 -
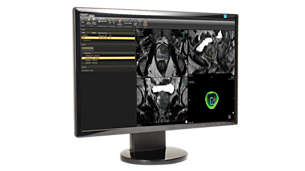
DynaCAD Prostate
Offers a comprehensive set of tools for real-time analysis, review, and reporting of multi-parametric, multi-vendor(3) MRI studies. Enhances productivity by transferring images directly from the MRI to DynaCAD and utilizing its robust, automatic post-processing tools and display the results in customized hanging protocols for analysis and reporting.
784029 -
DynaCAD Urology
DynaCAD Urology is a purpose build solution that empowers urologists with a dedicated set of tools for utilizing multi-parametric MR data in fusion biopsy workflows. It also provides a solution for managing patients’ biopsy data in urology.
784044
-
Compressed SENSE
Click here to learn more -
![MRI in-bore experience]()
MRI in-bore experience
Click here to learn more -
![Genomics Workspace]()
Genomics Workspace
Click here to learn more
Results you can measure
Our clinical solutions can enhance detection and improve the patient experience by reducing unnecessary biopsies and potentially reducing post-procedure complications. Digital pathology saves time and resources through streamlined collaborative features and case management tools.
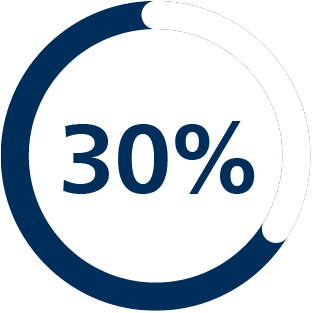
+30%
improvement in high-risk prostate cancer diagnosis using fusion biopsy vs standard biopsy5
-13.3%
reduction in pathological upgrading to clinically significant prostate cancer with targeted and systematic biopsies vs systematic biopsies alone5
+10%
increase in prostate cancers identified with combined targeted and systematic biopsy compared to systematic biopsy alone9
+19
hours/day saved through increased lab efficiency by using digital pathology workflows10
+50%
faster MRI scans using Compressed SENSE with virtually equal image quality11
Would you like to know more about how we can help your prostate cancer program?
Philips can assess where you are today so you can build an approach to improve prostate cancer diagnosis by integrating urology, radiology, pathology, genomics and oncology. Discover how we can help you implement, manage and grow your prostate care program.
Dive deeper
Related solutions
References
Results from case studies are not predictive of results in other cases. Results in other cases may vary.
1. Choi YH, et al. Comparison of cancer detection rates between TRUS-guided biopsy and MRI-targeted biopsy according to PSA level in biopsy-naive patients: a propensity score matching analysis. Clin Genitourin Cancer. 2019 Feb;17(1):e19-e25. doi: 10.1016/j.clgc.2018.09.007. Epub 2018 Sep 13. PMID: 30415878.
2. U.S. News and World Report: 2017 Best Hospitals Ranking (Cancer)
3. Yarlagadda VK, et al. MRI/US fusion-guided prostate biopsy allows for equivalent cancer detection with significantly fewer needle cores in biopsy-naive men. Diagn Interv Radiol. 2018 May-Jun;24(3):115-120. doi: 10.5152/dir.2018.17422. PMID: 29770762; PMCID: PMC5951198.
4. Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021.71:209-249. https://doi.org/10.3322/caac.21660
5. Siddiqui MM, et al. Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390-397.
6. Sonn GA, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013 Jan;189(1):86-91. doi: 10.1016/j.juro.2012.08.095. Epub 2012 Nov 14. PMID: 23158413; PMCID: PMC3561472.
7. Loeb S, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014 Jun;65(6):1046-55. doi: 10.1016/j.eururo.2013.12.062.
8. Rebbeck TR, et al. Temporal trends and racial disparities in global prostate cancer prevalence. Can J Urol. 2014 Oct;21(5):7496-506. PMID: 25347377; PMCID: PMC4955669.
9. Ahdoot M, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. NEMJ. 2020382:917–928. doi: 10.1056/NEJMoa1910038
10. Baidoshvili A, et al. Evaluating the benefits of digital pathology implementation: time savings in laboratory logistics. Histopathology. 2018 Nov;73(5):784-794. doi: 10.1111/his.13691. Epub 2018 Aug 13. PMID: 29924891.
11. Compared to Philips scans without Compressed SENSE.