I want to learn more
Stay up-to-date and get informed about interesting topics. Or get in touch with our sales department.
Addressing the obstacles to confident diagnosis:
Disease progression tracking
As new, personalized treatments are developed to slow disease progression, there is a vital need for imaging methods that can detect response to therapy at early follow-up times. The radiologist has an invaluable role in this process, as imaging provides direct insight into the various factors and treatments that delay or accelerate disease onset.
Here are some of the ways we are making disease progression (or regression) easier for radiologists to monitor.

What you'll see
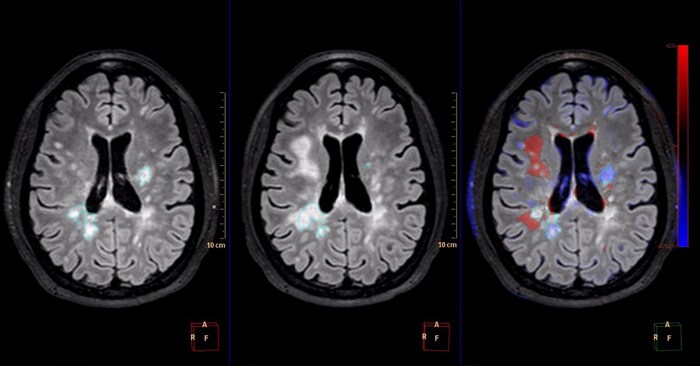
Comparative brain imaging
Tracking patient progression
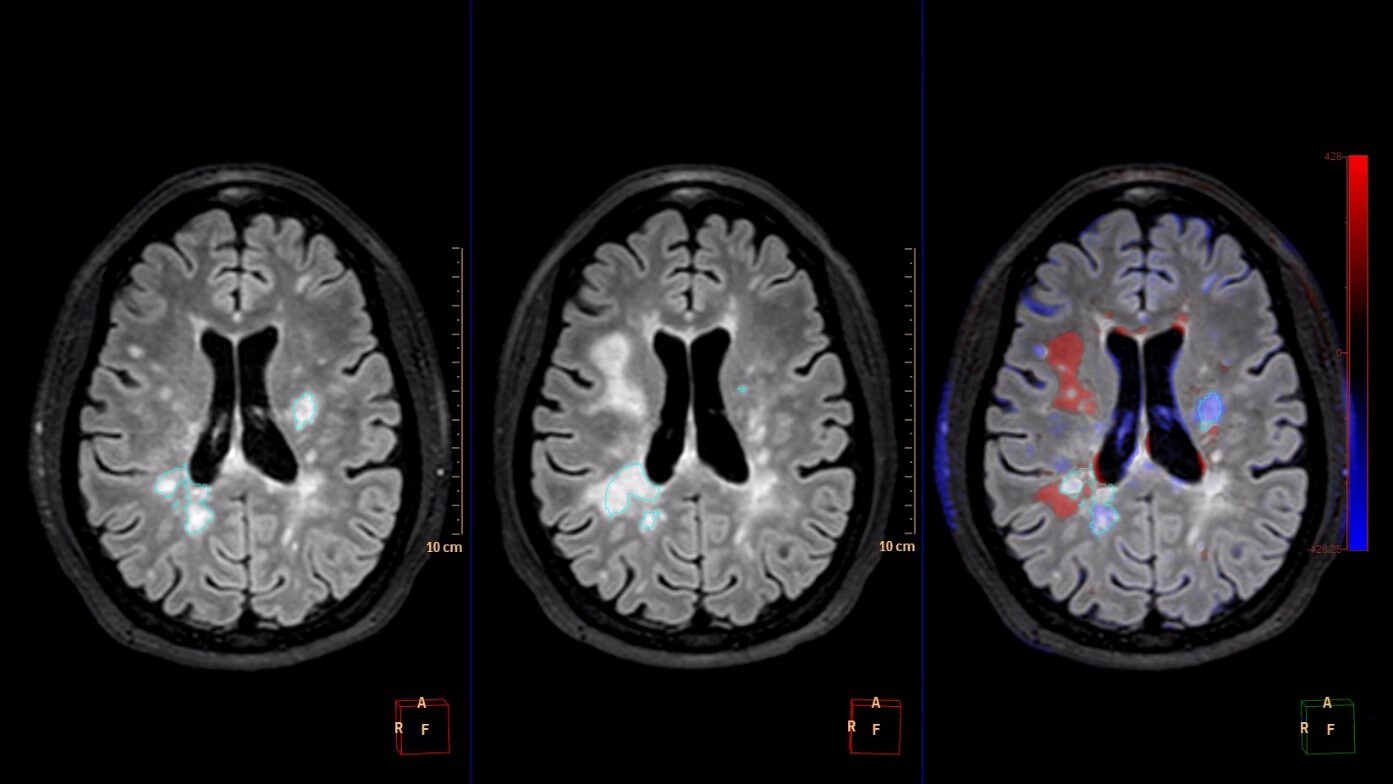
Radiologists are experiencing an increasing need for tools that better support the growing number of patients with neurological disorders such as dementia, stroke and multiple sclerosis. Critical to the understanding of these diseases and the development of effective treatments, is the radiologist’s ability to track their progression across patient groups, and the advancement of tools they have to support these efforts.
IntelliSpace Portal 9.0 offers Longitudinal Brain Imaging (LoBI), an application to help clinicians visualize subtle differences in the brain images of the same individual across time, which may be used by clinicians as the assessment of neurological disease progression. Multiple images are automatically aligned and registered to simplify comparison, and the application provides editing tools and volumetric quantification. Comparative Brain Imaging (CoBi) functionality even allows users to track subtle differences in the brain by subtracting scans taken at different points in time.
Technology that detects subtle differences in the brain over long periods of time may help in the assessment of neurological disease progression.
Tracking patient progression
Radiologists are experiencing an increasing need for tools that better support the growing number of patients with neurological disorders such as dementia, stroke and multiple sclerosis. Critical to the understanding of these diseases and the development of effective treatments, is the radiologist’s ability to track their progression across patient groups, and the advancement of tools they have to support these efforts.
IntelliSpace Portal 9.0 offers Longitudinal Brain Imaging (LoBI), an application to help clinicians visualize subtle differences in the brain images of the same individual across time, which may be used by clinicians as the assessment of neurological disease progression. Multiple images are automatically aligned and registered to simplify comparison, and the application provides editing tools and volumetric quantification. Comparative Brain Imaging (CoBi) functionality even allows users to track subtle differences in the brain by subtracting scans taken at different points in time.
Technology that detects subtle differences in the brain over long periods of time may help in the assessment of neurological disease progression.
Tracking patient progression
Radiologists are experiencing an increasing need for tools that better support the growing number of patients with neurological disorders such as dementia, stroke and multiple sclerosis. Critical to the understanding of these diseases and the development of effective treatments, is the radiologist’s ability to track their progression across patient groups, and the advancement of tools they have to support these efforts.
IntelliSpace Portal 9.0 offers Longitudinal Brain Imaging (LoBI), an application to help clinicians visualize subtle differences in the brain images of the same individual across time, which may be used by clinicians as the assessment of neurological disease progression. Multiple images are automatically aligned and registered to simplify comparison, and the application provides editing tools and volumetric quantification. Comparative Brain Imaging (CoBi) functionality even allows users to track subtle differences in the brain by subtracting scans taken at different points in time.
Technology that detects subtle differences in the brain over long periods of time may help in the assessment of neurological disease progression.
Brain tumor follow-up
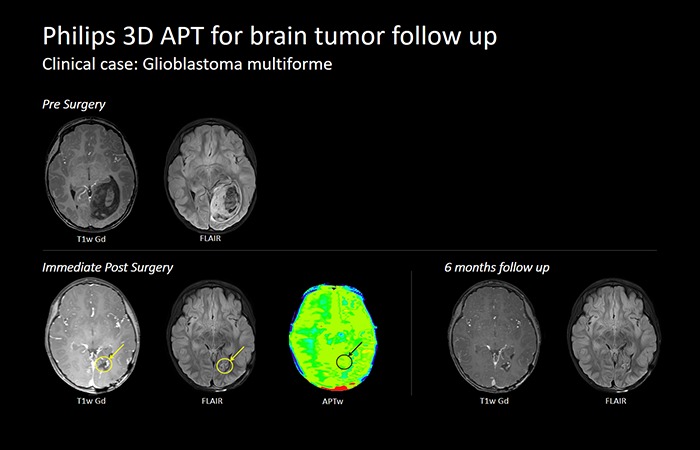
MRI serves as the current gold standard in tumor treatment response monitoring. However, prognostic information cannot be obtained until weeks after the initial treatment. It is crucial for radiologists to be able to differentiate recurrent tumors from treatment-induced changes such as radiation necrosis. This means there is a vital need for methods that can detect response to therapy at early follow-up times.
Through Philips 3D APT, the only molecular contrast for glioma tumor grading and treatment follow-up, radiologists can differentiate tumor progression from treatment effect. 3D APT uses the presence of endogenous cellular proteins to produce an MR signal that directly correlates with cell proliferation, a marker of tumor activity.
Through the support of Philips 3D APT and its ability to differentiate tumor progression from treatment effects, radiologists can now make improved clinical decisions for their patients.
CT success with low-contrast patients
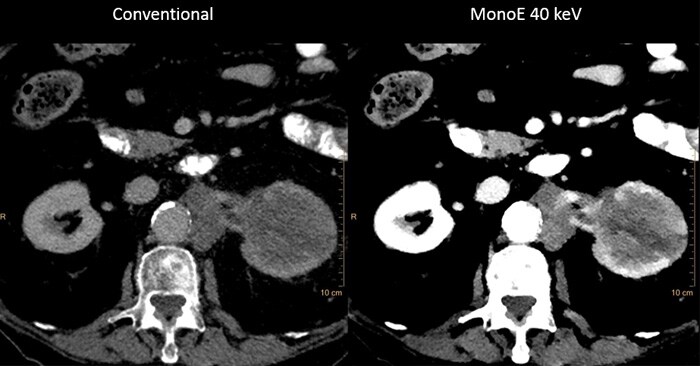
Addressing non-contrast CT
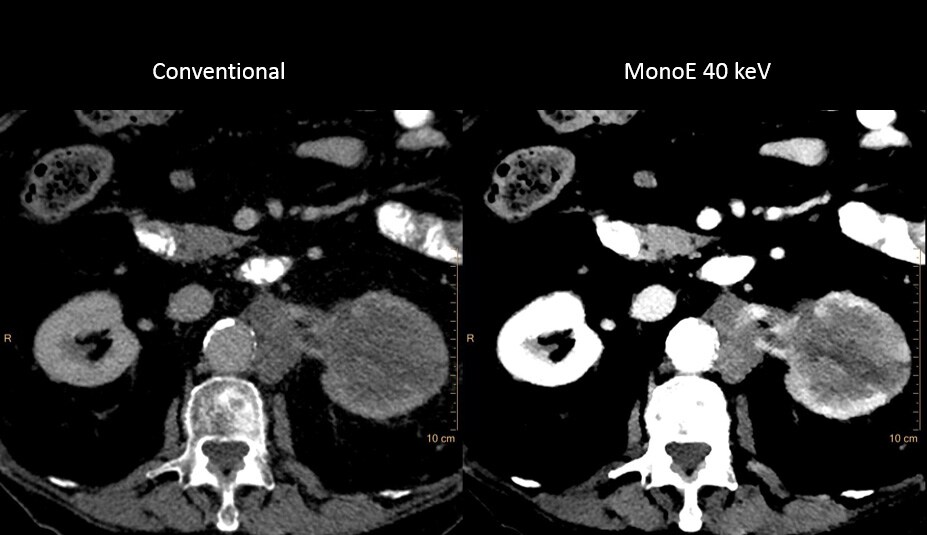
Roughly 14%* of the US population suffers from chronic kidney disease (CKD) and cannot tolerate typical contrast doses. These patients often require additional scanning, as they are scanned with non-contrast CT, which is then followed with other modality scans. These additional scans increase time and cost for patient and provider.
IQon Spectral CT is the world’s first and only detector-based spectral CT which captures spectral information every time a patient is scanned, at low-dose. IQon delivers diagnostic certainty using multiple layers of spectral data, allowing the first exam to be the right exam, and keeping workflow in the health system efficient.
IQon Spectral CT enables these patients to be scanned with low volumes of contrast. Spectral results 100% of the time eliminate the patient selection dilemma and remove the need for timely and costly additional exams.
*National Institutes of Health. Kidney Disease Statistics for the United States. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease.
Tracking disease progression across modalities
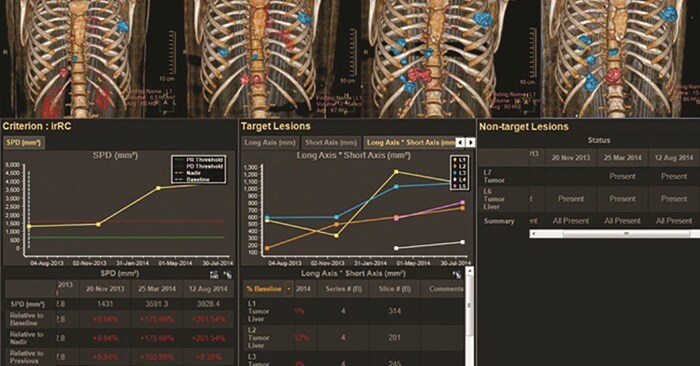
Measuring change in lesion diameter
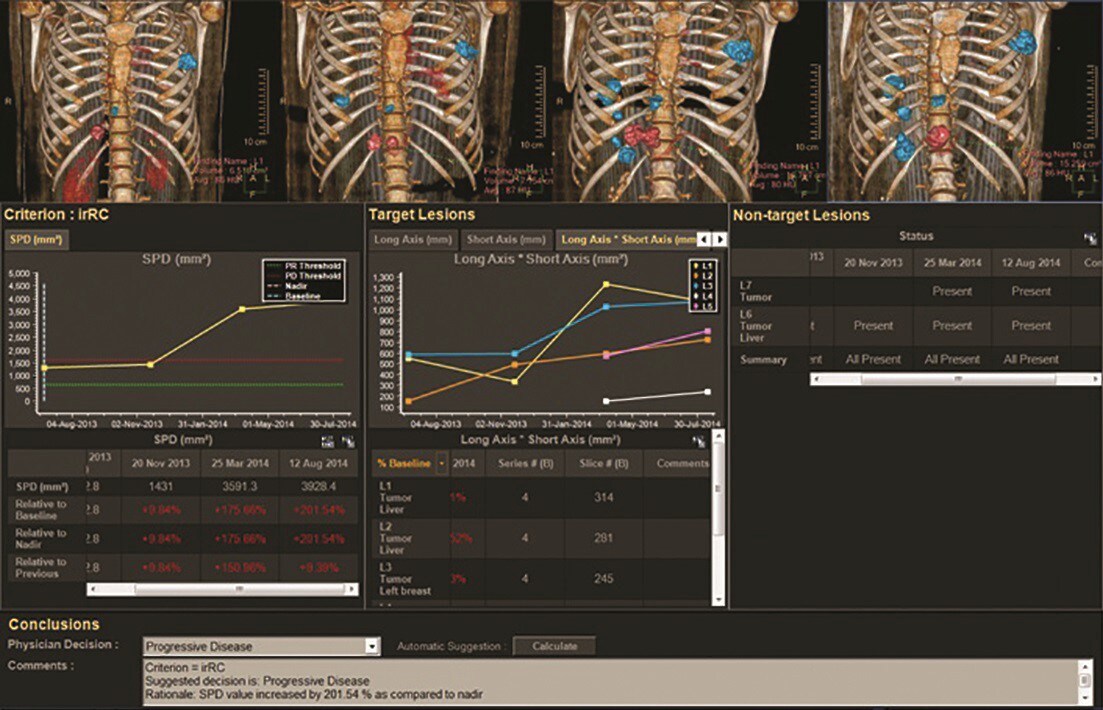
Changes in lesion size in response (or lack of response) to treatment is often measured manually on PACS systems and dictated in medical records with a reference to the comparison date. Although this standard was designed to be simple, the attempt to implement the Response Evaluation Criteria in Solid Lesions (RECIST) with manual PACS or similar methods by locating and measuring lesions in a follow-up exam can be time consuming. Computing percentage change in lesion diameter is often calculated using a spreadsheet or a hand-held calculator.
The Philips IntelliSpace Portal’s automated Multi-Modality Lesion Tracking (MMTT) application provides features and tools to decrease the time required to implement RECIST into disease progression tracking, as well as other standard and emerging response criteria. Baseline and follow-up images from multiple modalities can be loaded into the MMTT application, where the RECIST criteria – based on percent change in lesion diameter – is then calculated by the system in a results screen that shows physicians treatment response categorization.
Enabling an average total time savings of 6:17 minutes per evaluation in comparison with PACS, the Multi-Modality Lesion Tracking application may lead to frequent use of imaging response criteria, facilitating enhanced care management for cancer patients.
Measuring change in lesion diameter
Changes in lesion size in response (or lack of response) to treatment is often measured manually on PACS systems and dictated in medical records with a reference to the comparison date. Although this standard was designed to be simple, the attempt to implement the Response Evaluation Criteria in Solid Lesions (RECIST) with manual PACS or similar methods by locating and measuring lesions in a follow-up exam can be time consuming. Computing percentage change in lesion diameter is often calculated using a spreadsheet or a hand-held calculator.
The Philips IntelliSpace Portal’s automated Multi-Modality Tumor Tracking (MMTT) application provides features and tools to decrease the time required to implement RECIST into disease progression tracking, as well as other standard and emerging response criteria. Baseline and follow-up images from multiple modalities can be loaded into the MMTT application, where the RECIST criteria – based on percent change in lesion diameter – is then calculated by the system in a results screen that shows physicians treatment response categorization.
Enabling an average total time savings of 6:17 minutes per evaluation in comparison with PACS, the Multi-Modality Tumor Tracking application may lead to frequent use of imaging response criteria, facilitating enhanced care management for cancer patients.
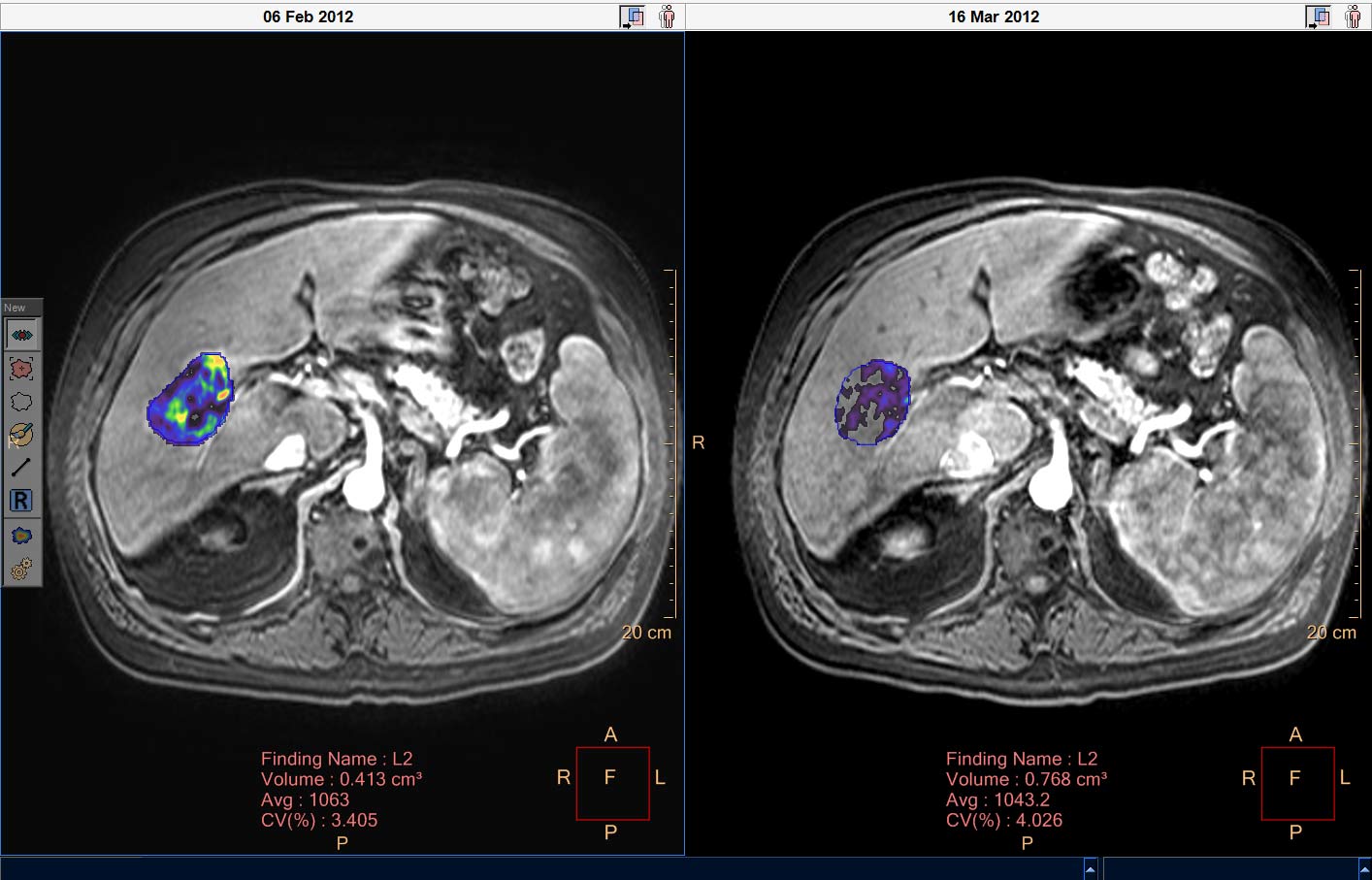
Liver lesion follow-up
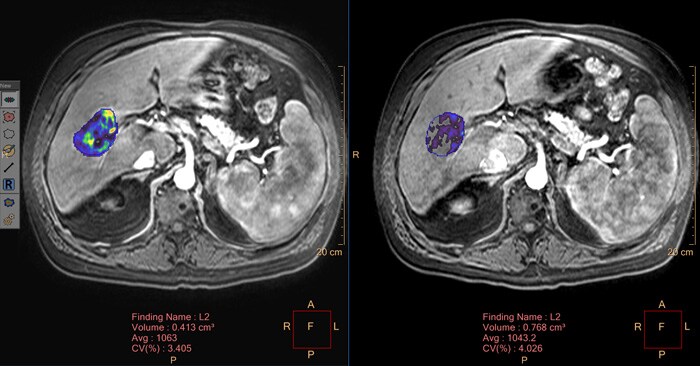
Measurement of potentially viable liver lesion tissue
In mid-to-late stage cancer diagnoses especially, patient treatment is comprised of minimally invasive, catheter-based, image guided lesion therapies, making the advancement of lesion assessment technology critically important. Traditional treatment response tracking methods are purely based around the measurement of lesion size and often don’t consider lesion viability or cell death, features that can be indicative of treatment response in patients with liver cancer.
qEASL (quantitative European Association for the Study of the Liver) is a post-processing tool that allows radiologists to make a standardized analysis of 3D imaging scans to obtain a measurement of potentially viable liver lesion tissue. It provides a visual indication by a color map overlayer on the scans. The color regions of the segmented lesions are where there is more enhancement than the pre-defined reference region. qEASL has been fully integrated into Philips’ Multi-Modality Lesion Tracking application within the IntelliSpace Portal.
The results obtained may be used as a tool by clinicians in determining the diagnosis of patient disease conditions in various organs, tissues, and other anatomical structure.
Learn how Philips solutions help radiologists overcome obstacles to confident diagnosis. See them all:
I want to learn more